1.3 COMPOSITION OF SOILS
1.3.1 Soil Formation
Soils form when rocks break down over time through processes called weathering. There are two main types of weathering:
- Physical Weathering: This is when rocks break into smaller pieces without changing their chemical makeup. Processes like exfoliation, unloading, erosion, freezing, and thawing help break the rock into smaller fragments.
- Chemical Weathering: Here, the rock not only breaks into smaller pieces but also changes chemically. This happens because of reactions like hydration (water combining with minerals), carbonation (reaction with carbon dioxide), and oxidation (reaction with oxygen).
Often, both physical and chemical weathering work together. When soils form in the same place where the rock breaks down, they are called residual soils because they keep many of the original rock elements. But if water moves these particles away, like in a river, the soil is known as alluvial (or fluvial) soil, and it often forms in layers.
1.3.2 Soil Types
Soils are made up of different types of particles that give them their texture. We can group them by size:
- Gravels – Big particles that you can often see with the naked eye.
- Sands – Medium-sized particles that feel gritty when you rub them between your fingers.
- Silts – Finer particles that feel smooth.
- Clays – Very fine particles that can be sticky and moldable when wet.
Sands and gravels are known as coarse-grained soils, while silts and clays are called fine-grained soils. Coarse-grained soils are usually classified by knowing the range of particle sizes. For fine-grained soils, we also need to know which minerals they contain because that affects how they behave when a load is applied.
Special soil types have their own names. For instance, calcareous soils fizz when mixed with acid because they contain calcium carbonate, and glacial soils come from ice-age deposits that mix different sizes of particles.
1.3.3 Soil Minerals
Minerals are the building blocks of soils. They are crystalline solids that come together to form the soil’s structure. Most of the minerals of interest to engineers are made mostly of oxygen and silicon.
In coarse-grained soils, the most common mineral is quartz. Quartz is hard and forms particles that are naturally angular, though weathering by water can round them off over time.
Fine-grained soils, such as clays, are made up of tiny, plate-like particles. The main minerals in these clays are:
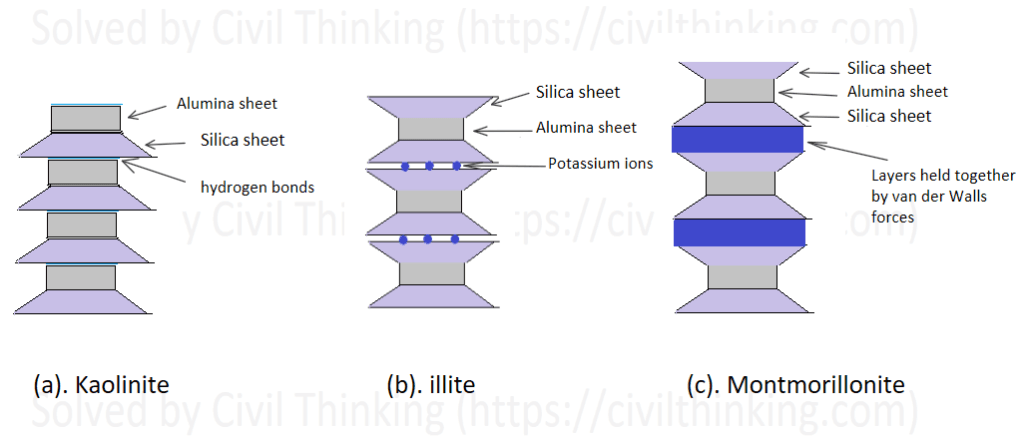
- Kaolinite: This mineral consists of one silica sheet bonded with one alumina sheet, forming a layer about 0.72 nm thick. These layers are held together by hydrogen bonds. (Refer to Figure 1.2a)
- Illite: Illite has layers arranged as one alumina sheet sandwiched between two silicate sheets, each layer being about 0.96 nm thick. The layers are held together by potassium ions. (Refer to Figure 1.2b)
- Montmorillonite: Similar to illite in structure but with layers held together by weak van der Waals forces. This allows water to get between the layers, making the soil swell. If sodium (Na+) is the main exchangeable ion, there is typically one water layer; if calcium (Ca2+) is dominant, there are usually two water layers. Montmorillonite is often called a swelling or expansive clay. (Refer to Figure 1.2c)
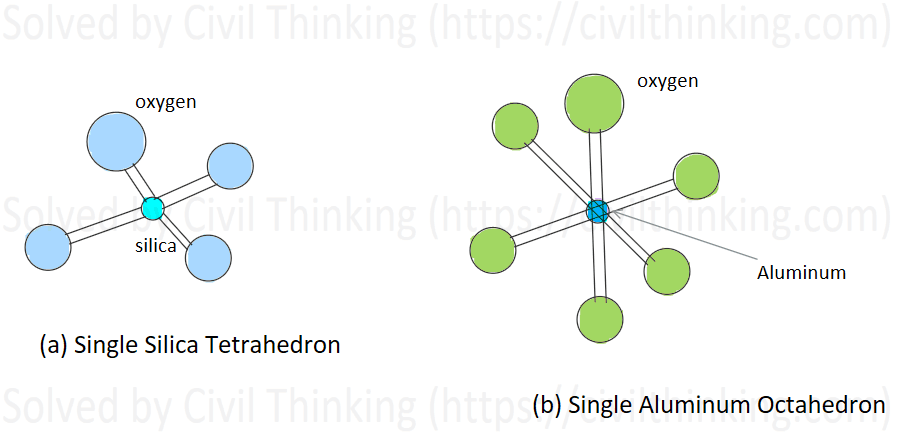
For extra clarity, consider the silica tetrahedron – a tiny pyramid with one silicon atom at its center and four oxygen atoms at the corners. These tetrahedrons link together by sharing oxygen atoms, forming continuous sheets. In the process of forming clay minerals, these silicate sheets may bond with layers formed by aluminum and oxygen with hydroxyl groups; these additional layers (often referred to as alumina layers) result from aluminum atoms coming together in an octahedral arrangement. This bonding creates a stable, layered structure that is essential in defining the properties of clay. (See Figures 1.1a and 1.1b)
In summary, this section explains how soils form from rock weathering, describes the different types of soil based on particle size, and discusses the minerals that make up soils. This knowledge is key for understanding how soils behave under weight and why they are important in construction and engineering.
Below are the diagrams: Fig. 1.1a, 1.1b, Fig. 1.2a, 1.2b, and Fig. 1.2c.
1.3.4 Surface Forces and Adsorbed Water
When we break a solid into smaller pieces, its overall surface area increases a lot compared to its volume. For example, a 1 cm cube has a surface area of 6 cm², but if we cut it into many small 1 mm cubes, the total surface area grows to 60 cm². This concept is very important in soils.
Different soils have very different specific surface areas (surface area per unit mass). For instance, sands have about 0.01 m² per gram, while clays like montmorillonite can have up to 1000 m² per gram. Kaolinite and illite, two common clay minerals, have specific surfaces of 10–20 m² and 65–100 m² per gram respectively. To put it in perspective, the surface area of just 45 grams of illite is as big as a football field!
The huge surface areas in fine-grained soils (like clays) make surface forces much more important in their behavior than in coarse-grained soils (like sands). These forces affect how much water the soil can hold in tiny spaces between particles.
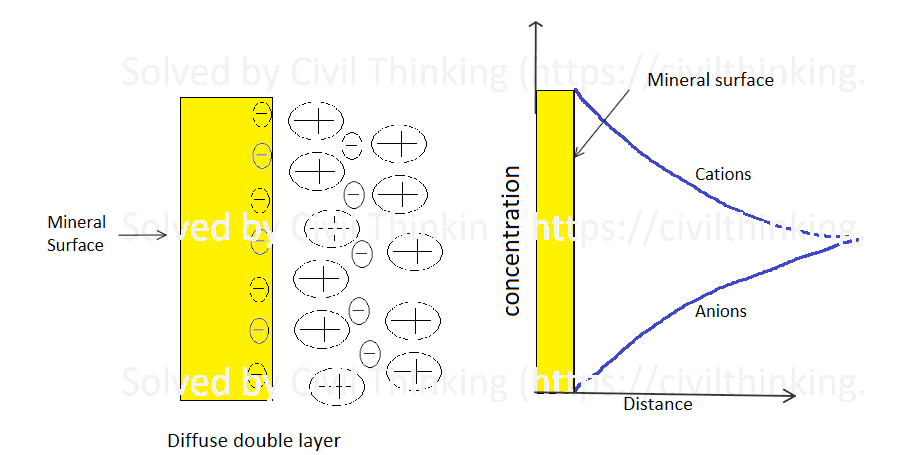
The particles of fine-grained soils carry negative charges on their surfaces. These negative charges attract positively charged particles (cations) and the positive side of water molecules. As a result, a thin film of water—known as adsorbed water—is firmly bonded to the surfaces. This thin layer is also called the diffuse double layer.
There are two main types of surface forces at work:
- Attracting Forces: These come from London–van der Waals forces. They act over longer distances and become weaker as the distance between particles increases.
- Repelling Forces: These are due to the diffuse double layer. Each particle has an ionic cloud around it; when these clouds overlap as particles come close, the negative charges repel each other.
Even when soils are dried in an oven (typically at 105 ± 5°C), the adsorbed water remains. This water plays a key role in the soil’s properties—for example, it contributes to soil plasticity, which will be discussed further in Chapter 4. In addition, the way this water interacts with the soil is crucial for understanding how toxic chemicals move through and can be removed from soils.
Below are the referenced diagrams: Fig. 1.3.